Disclaimer: This will only cover the SL content of the course.
Fundamentals of organic chemistry
Organic chemistry is the field in chemistry which studies carbon compounds.
Carbon is able to combine with many different molecules including hydrogen which make up the basis of organic life. They can combine to make polymer chains, made up of c-c bonds, through a process called catenation
Cyclic: The carbon chain links back to itself to form a ring shaped structure
Degrees of saturation
Saturated compounds contain only single bonds whereas Unsaturated compounds contain double or triple bonds. Only unsaturated compounds can react.
Homologous series
A homologous series is a series of compounds all of the same family which each differ by on structural unit. This allows us to group together the main compounds which exist in chemistry, into groups which have similar physical properties.
Different homologous series
- Alkanes
- Alkenes
- Alkynes
- Halogenoalkanes
- Alcohol
- Ether
- Aldehyde
- Ketone
- Arene
- Carboxylic acid
- Ester
- Amine
- Amide
- Nitrile
Trends in boiling points
For each successive structural unit, there will be a progressive increase in surface area and molecular mass. These are the factors which affect boiling point, therefore increasing these leads to an increase in boiling point
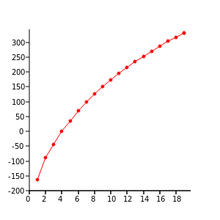
Plotting number of carbon atoms vs boiling points gives a curved trendline, which is known in maths as the harmonic series. The reason it takes this shape is because each increase in structural unit there is a proportionally smaller effect on boiling point.
Effect of branches on boiling point
A greater number of branches decreases the surface area, leading to a lower boiling point. For example a cyclic shaped compound should have a lower boiling point than one of a single chain.
Distinction between Empirical, Molecular and structural formula
Empirical formula: This is the ratio of the different elements which make up a compound. It does not give the structure or actual number of elements.
Molecular formula: This is the number of atoms of each element which make up a compound. This does not give structure.
Structural formula: This shows the arrangement of atoms in the element. Depending on the type of formula it can be presented either graphically or as text.
types of Structural formula
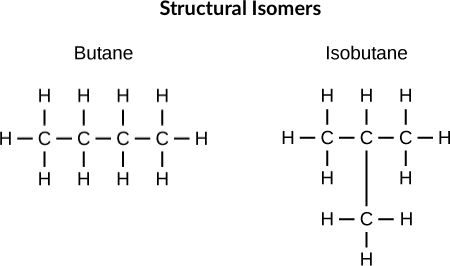
Isomerism
Structural Isomers are compounds with exactly the same molecular formula, but with different arrangements of the elements in the compound.
You can have:
- Positional isomers - Functional groups in different positions.
- Chain isomers - Different branching in carbon chains.
- Functional group isomers - Functional groups are completely different
Functional groups
Classes: These are the different types of compounds in organic chemistry. This is synonymous with "homologous series"
Functional groups: This is the reactive part of the molecule. It is responsible for the characteristic reaction of the different homologous series
Naming organic molecules (IUPAC)
Essentials for naming
- Root of name - indicates length carbon chain (meth, eth, prop, but, pent, hex)
- Ending of name - This indicates degree of saturation ( -An=Single, -En= double, -yn = Triple)
- Prefix or suffix - These indicate presence of functional groups
- Prefixes: hydroxyl- =OH, fluro-= F
- Suffixes: -Ol = OH, -Oic acid = COOH
Detailed naming rules
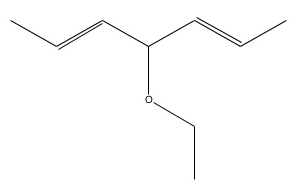
Note: For the examples we will refer to the complicated looking ether above. (Don't worry you're unlikely to get one this difficult in the exam)
| # |
What to identify |
Naming convention |
Example |
| 1 |
Identify the parent carbon chain, which is the one which is longest |
The length gives the root of the name.(meth, eth, prop, but, pent, hex, hept)
If two are the same length pick the one with the most side branches.
|
- The longest chain is 7 carbons long, so the prefix is hept-
hept-
|
| 2 |
Identify degrees of saturation e.g single, double, triple bounds. |
This determines the part after the root. (-an, -en ,-yn) |
The parent chain has two double bond so the suffix is -en.
hepten
|
| 3 |
Identify any functional groups |
This determines the suffixes/prefixes. Only use a prefix if you can't use a suffix.
- OH: -ol (suffix)
- COOH: -oic acid (suffix)
- CH:- e (suffix)
- OC: oxy- (prefix)
- NH2-amine (suffix)
|
There is an oxygen atom so there is an ether (OC) functional group. There is also an alkyl group, Thus we add oxy- and -e
The ether group is part of a main chain of two carbon length so we also prepend eth-
ethoxyheptene
|
| 4 |
identify carbon side chains to main chain |
Add the names of the carbon chains as prefixes. These have the root which tells the number of carbons and the suffix which tells if their are double, or triple bonds. Suffixes: single= yl, double=enyl: triple = yenyl) |
There are no side chains.
|
| 5 |
Check if prefixes are in the right order |
If there are multiple prefixes they must be ordered alphabetically. |
They are already in alphabetical order, in this case. |
| 6 |
Check if there is multiple of the same functional group/side chain/degrees of saturation |
If there are multiples add prefix -di, -tri, -tetra to show how many |
There are two double bonds so we add di- before the -en.
ethoxyhepta-di-ene (we add "a" because the primary suffix is followed by a constant)
|
| 7 |
Number carbon chain and then use the numbers to locate the side chains/functional groups/degrees of saturation |
The numbering chosen should be the one which results in the lowest total of the locations. |
- The ether group is on the 4th carbon from right or left
- The double bonds are on the 2nd and 5th carbon
- So name becomes 4-ethoxyhepta-2,5-diene
|
| 8 |
Exception to rule 7: If a functional group is present and must be at the end of the chain, then numbering must begin at this functional group. |
|
|
Benzene
This is a compound with the molecular formula \(C_6 H_6 \) Benzene is cyclic and forms a hexagon structure, where each carbon has one hydrogen. Each carbon has one double bond to another carbon and also one single bond.This creates a resonance structure

In skeletal form, to show the resonance structure, we put a circle inside the hexagon chain. This shows that the double bonds location can be in different places, hence the bond order is 1.5
Primary, secondary and tertiary compounds
Primary, secondary and tertiary are ways of classifying organic compounds
Alcohols and Halogenoalkanes
Whether an alcohol/halogenoalkane is primary, secondary or tertiary depends on the number of carbons which are bonded to the carbon which is bonded to the hydroxyl group/halogen
| Classification |
Requirement |
Diagram |
| Primary |
One carbon bonded to the carbon bonded to the hydroxyl/halogen |
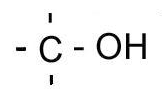 |
| Secondary |
Two carbons bonded to the carbon bonded to the hydroxyl/halogen |
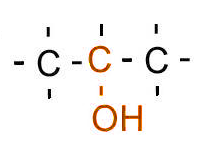 |
| Tertiary |
Three carbons bonded to the carbon bonded to the hydroxyl/halogen |
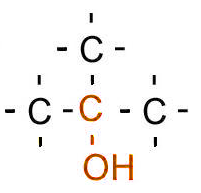 |
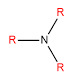
Amines
Whether an amine is primary, secondary or tertiary depends on the number of alkyls which are bonded to the nitrogen.
| Classification |
Requirement |
Diagram |
| Primary |
One alkyl bonded to the nitrogen |
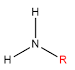 |
| Secondary |
Two alkyls bonded to the nitrogen |
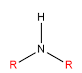 |
| Tertiary |
Three alkyls bonded to the nitrogen |
 |
Functional group reactions
Alkanes
These are saturated hydrocarbons containing only carbon-carbon and carbon-hydrogen bonds.
Reactivity of alkanes
Alkanes have low reactivity for two reasons:
- Molecules are nonpolar
- C-C and C-H are relatively strong and require a high activation energy to break.
Combustion reactions of alkanes (oxidisation)
Complete combustion: This is when alkanes are burnt in excess oxygen, to produce \(CO_2\) and \(H_2 O\). The (unbalanced) chemical equation is: \(Alkane_{(g)} + {O_2}_{(g)} \longrightarrow {CO_2}_{(g)} + {H_2 O}_{(l)}\)
incomplete combustion: This is when alkanes are burnt with limited oxygen, to produce \(CO_2\) , \(H_2 O\) and \(CO\) or \(C\) . The (unbalanced) chemical equation is either \(Alkane_{(g)} + {O_2}_{(g)} \longrightarrow CO_{(g)} + {H_2 O}_{(l)}\) or \(Alkane_{(g)} + {O_2}_{(g)} \longrightarrow C (s) +{ H_2 O}_{(l)}\)
Free radical substitution
Free radical: An atom with unpaired electron which free to bond. The free radical is represented by a dot
Homolytic fission:splits the diatomic to produce two free radicals, with the same (homo) charge
This is a process where a halogen becomes a free radical and then substitutes for a hydrogen in the alkane. This process is a chain reaction and has three stages: initiation, propagation and termination.
- Initiation: A Halogen diatomic undergoes homolytic fission, which means it splits the diatomic to produce two free radicals. This is caused by UV light.
- Propagation: The free radical is very reactive and so can strip a hydrogen of the alkane. Forming a \(C-H\) molecule. The alkane is now a free radical and can cause other chlorine molecules to undergo homolytic fission. This creates a chain reaction
- Termination: The reaction terminates after all the free radicals come together to form stable molecules. This can happen in three ways:
- Chlorine radical and alkane radical come together to form a halogenoalkane
- Two chlorine radicals join together to form a \(Cl_2\) molecule
- Two alkane radicals join to form a longer alkane.

Alkenes
Reactivity of alkenes
Alkenes are more reactive than alkanes because they have unsaturated double bonds. This means that the double bond can become a single bond leaving space for the addition of other molecules. This is called an addition reaction.
Addition reactions
This is a reaction where the double bond is converted to a single bond allowing for the addition of other molecules.
In the IB course there are three different types of addition reactions covered:
- Halogenation: Addition of a halogen or hydrogen halide (e.g HCL) to an alkene to form a halogenoalkane..
- Hydrogenation: Addition of a hydrogen to an alkene. Has high activation energy so nickel catalyst and/or high temperature and pressure is needed.
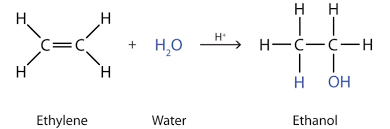
- Hydration: Addition of water to an alkene to form an alcohol. This process has a high activation energy and requires a strong catalyst like sulfuric acid. It is reversible, so to form alcohol high pressure is needed.
Addition polymerisation
Addition polymerisation is when the alkene combines with other alkenes through an addition reaction to form long chains called polymers. Polymers are very important because they are used to create plastics such as PVC of PTFE.

Monomer: The alkene which undergoes polymerisation.
Repeating unit: this a part of the polymer which, when repeatedly combined, would form the full polymer. (excluding the end units).
Polymer: A long chain of monomers. Represented by the repeating unit surrounded by a bracket with a n in the bottom-right corner.
Alcohols
Combustion of alcohols
Like alkanes alcohols can undergo combustion reactions. You need to know how to write the equations for complete combustion, which is easy if you know that the products are again \(CO_2\) and \(H_2 O\)
\(C_2 H_6 O + 3O_2 \longrightarrow 2 CO_2 + 3H_2O\)
Oxidation reactions
Alcohols can be oxidized into other functional groups, depending on whether they are primary, secondary or tertiary. An oxidizing agent such as potassium dichromate (K2Cr2O7) is used as oxygen does not split easily.
- Primary alcohols undergo a two step process. Alcohol → Aldehyde → Carboxylic acid.
- Secondary alcohols form ketones.
- Tertiary alcohols have no hydrogen atoms bonded to the carbon atom directly bonded to the hydroxyl so they won't oxidize.
Obtaining aldehydes vs acids
To obtain an aldehyde from oxidizing a primary alcohol instead of a carboxylic acid the aldehydes must be removed from the reaction as soon as they are formed to prevent them oxidizing further. This can be done by taking advantage of the aldehydes lower boiling point compared to the alcohol. Here the alcohol is added to a boiling oxidizing agent so that as soon as the aldehyde forms it boils and can't be oxidized any further. Then a liebig condenser (Long tube with cold water) is add so that when the aldehyde gas passes through it, it will condense and can be collected.
The carboxylic acid can be obtained naturally, but to speed it up a boiling oxidizing agent is used and then a reflux condenser is added so that the evaporated aldehyde condense and goes back to the oxidizing agent, until it is fully oxidized.
Esterification
Esterification is when an alcohol and a carboxylic acid combine to form an ester. This reaction is a subcategory of condensation reactions, which are reaction that involve two molecules joining to form a large molecule. These reactions have steam as a by-product which is why they are called condensation reactions.
To combine the two molecules the Hydrogen at the end of the carboxylic acid and the OH from the alcohol are removed by a strong catalysts, so that they can join.
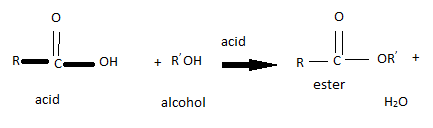
Note: Esters are volatile and are nice smelling. For this reason they are often used in perfumes or artificial flavourings
Halogenoalkanes
Nucleophile: a species with a lone pair of electrons and is thus attracted to positive species
In Halogenoalkanes the electronegative halogen forces the connecting carbon to have a positive charge. This makes it possible for Nucleophiles to replace the halogen, in a process called nucleophilic substitution.
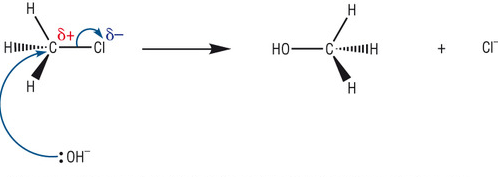
(you don't need to memorize this diagram but it helps to visually understand nucleophilic substitution)
Equation which might come up: 
Benzene
Electrophilic substitution
Electrophile: A positively charged species which is attracted negative species.
In benzene, the ring structure gives it areas of high electron density above and below (why this happens is HL knowledge). Electrophiles are attracted to these negatively charged areas and can replace the hydrogens in benzene. This is electrophilic substitution
Evidence for resonance in benzene
- We know benzene has resonance because it does not undergo addition reaction with halogens (halogenation), which suggests it has high stability. For this to be the case it cannot have double bonds otherwise they would be able to be broken and perform addition reactions like the alkenes do.
- Benzene, seen under a microscope, is perfectly symmetrical which would not be possible if there were some single bonds and some double bonds. This suggests that all bonds are equal which is only possible with resonance
View count: 46832